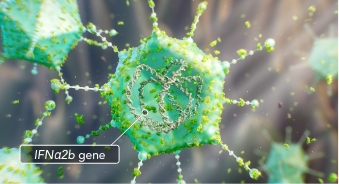
ADSTILADRIN (nadofaragene firadenovec-vncg) is a non-replicating adenoviral vector that delivers the human interferon alpha 2B (IFNα2b) gene to bladder urothelial cells.1

MECHANISM OF ACTION
ADSTILADRIN Offers a Unique Way
to Treat NMIBC as a Monotherapy
ADSTILADRIN is a non-replicating adenoviral vector–based gene-mediated immunotherapy
 1
1
 2
2
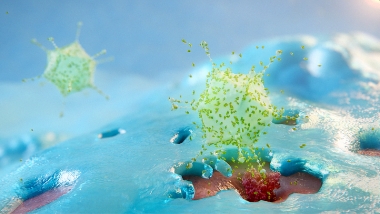
Once inside the bladder, ADSTILADRIN penetrates the bladder urothelial cells and travels to the cell nucleus to deliver the IFNα2b gene.1
 3
3
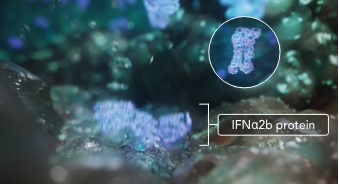
Both normal urothelial and tumor cells in the bladder that have taken up ADSTILADRIN begin to produce and secrete high and transient local expression of IFNα2b, a naturally occurring cytokine.1,2
IFNα2b has antitumor activity and enhances the body’s
natural ability to fight cancer1,2
The bladder is an ideal organ for gene therapy
Key reasons the bladder is ideal for this type of therapy:
- Direct contact between the gene vector and tumor cells3,4
- Local administration limiting systemic exposure4
- Easy access to monitor effects of therapy5
Gene therapy helps fight cancer by:
- Bolstering an immune response5,6
- Protecting healthy cells from the side effects of these treatments5
Abbreviation: NMIBC, non–muscle-invasive bladder cancer.
Sign Up to Receive Information and Updates on ADSTILADRIN (nadofaragene firadenovec-vncg)
Form ToggleReferences: 1. ADSTILADRIN. Package insert. Ferring Pharmaceuticals, Inc; 2023. 2. Medrano RFV, Hunger A, Mendonça SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017;8(41):71249-71284. doi:10.18632/oncotarget.19531 3. Narayan VM, Dinney CPN. Intravesical gene therapy. Urol Clin North Am. 2020;47(1):93-101. doi:10.1016/j.ucl.2019.09.011 4. Mahendran R, Tham SM, Esuvaranathan K. Gene therapy in urology. InTech. 2011. doi:10.5772/22235 5. Amer MH. Gene therapy for cancer: present status and future perspective. Mol Cell Ther. 2014;2:27. doi:10.1186/2052-8426-2-27 6. Das SK, Menezes ME, Bhatia S, et al. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230(2):259-271. doi:10.1002/jcp.24791
Important Safety Information
INDICATION
ADSTILADRIN is a non-replicating adenoviral vector-based gene therapy indicated for the treatment of adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: ADSTILADRIN is contraindicated in patients with prior hypersensitivity reactions to interferon alfa or to any component of the product.
WARNINGS AND PRECAUTIONS:
- Risk with delayed cystectomy: Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. If patients with CIS do not have a complete response to treatment after 3 months or if CIS recurs, consider cystectomy.
- Risk of disseminated adenovirus infection: Persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus. Avoid ADSTILADRIN exposure to immunocompromised or immunodeficient individuals.
DOSAGE AND ADMINISTRATION: Administer ADSTILADRIN by intravesical instillation only. ADSTILADRIN is not for intravenous use, topical use, or oral administration.
USE IN SPECIFIC POPULATIONS: Advise females of reproductive potential to use effective contraception during ADSTILADRIN treatment and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during ADSTILADRIN treatment and for 3 months after the last dose.
ADVERSE REACTIONS: The most common (>10%) adverse reactions, including laboratory abnormalities (>15%), were glucose increased, instillation site discharge, triglycerides increased, fatigue, bladder spasm, micturition (urination urgency), creatinine increased, hematuria (blood in urine), phosphate decreased, chills, pyrexia (fever), and dysuria (painful urination).
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch or call 1-800-332-10881-800-332-1088. You may also contact Ferring Pharmaceuticals at 1-888-FERRING1-888-FERRING.
Please see full Prescribing Information for ADSTILADRIN.
Are you a US healthcare professional?
ADSTILADRIN ASP UPDATE
Effective April 1, 2024, ADSTILADRIN has established an average sales price (ASP) in accordance with the guidelines set forth by the Centers for Medicare & Medicaid Services Part B. This determination is a significant milestone for Ferring Uro-Oncology and represents our commitment to providing transparency and value to our customers.
To get ongoing updates about ADSTILADRIN, please sign up here.